By Kayladanesh – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=79592408
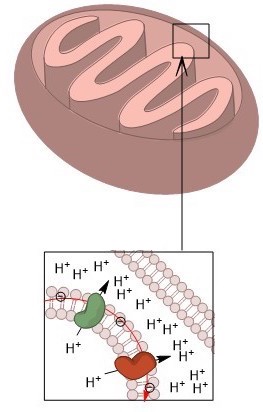
A mitochondria works like a battery. It maintains a voltage gradient across its inner-membrane. The outside of the membrane has a positive charge and the inside has a negative charge. Protons from the outside flow back through the membrane down the gradient, releasing energy, through a large cross-membrane protein complex called ATP synthase, which uses that energy to create ATP.
The energy to maintain the voltage gradient is obtained by oxidizing hydrocarbons – fats and proteins. Whether the caloric source is fat or protein, most of the hydrocarbons are oxidized in the mitochondria. Glucose is broken down to a compound called pyruvate in the cell before entering the mitochondria. Fats go through a process called beta oxidation which breaks them down into Acetyl-Coenzyme A. Both pyruvate and Acetyl-CoA enter into the krebs cycle to be completely oxidized. We are fire in a bottle.
The main product of the oxidation are the reduced electron carriers NADH and FADH2. Remember, oxidation is just electrons flowing from carbon and hydrogen to oxygen. In biological “redox” systems, instead of the electrons flowing through a wire, like from the negative to the positive terminal of a battery, they are transferred from molecule to molecule. Each time they are transferred, a little energy is given off.
By CNX OpenStax – http://cnx.org/contents/GFy_h8cu@10.53:rZudN6XP@2/Introduction, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=49924807
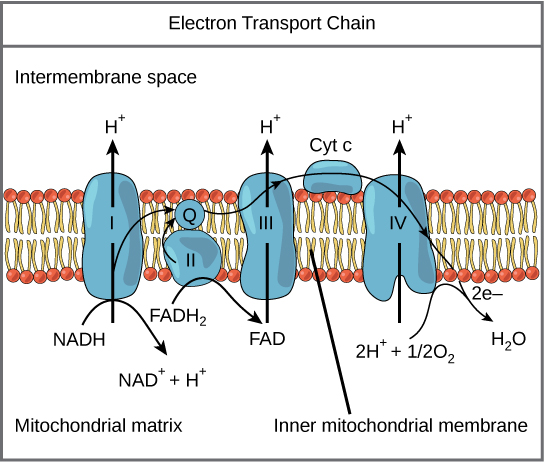
NADH and FADH2 store the energy from the oxidized hydrocarbons and deliver them to Complex I and II of the electron transport chain. From there the electrons follow the thin black arrows in the diagram. They are passed from Complex I and II to Coenzyme Q to complex III to Cytochrome C to complex IV before returning to the mitochondrial matrix to form water – Oxygen finally gets its electrons back. Along the way, protons are pumped by complexes I, III and IV.
In the final step, protons move through complex V, aka ATP synthase, back into the mitochondria. The released energy from the protons moving down the voltage gradient is used to phosphorylate a molecule of ADP to ATP. ATP is used to power most of our bodily processes like moving our muscles. Here’s an amazing video of the process that is part of the amazing Wikimedia Commons media project.
By Cwallworklegal – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=79116702

Mitochondria is plural; the singular is mitochondrion.
Robin and totaram, you are likely correct, but I’m probably going to keep saying it the same way.
Just being pedantic: Mitochondrion is singular if I am not mistaken (non-English speaking person here). Have to work through the rest more diligently.