An oversimplified version of the idea behind The ROS Theory of Obesity is that a diet high in saturated fat will cause “physiological insulin resistance” in your fat cells and they will fail to store fat. This will make you lean in time. When I first posted The Croissant Diet, my assumption was that trying the diet might elevate your blood sugar after meals and possible even the next morning, but that would be OK because you’d have regained control over your insulin signalling due to ROS generation in the mitochondria.
Based on the feedback from many who have tried the diet, I was clearly wrong about that. There have been some to report higher blood sugar by adding carbs back to a low carb diet, but even more people have reported a DROP in blood glucose levels, both immediately after meals and the next morning.
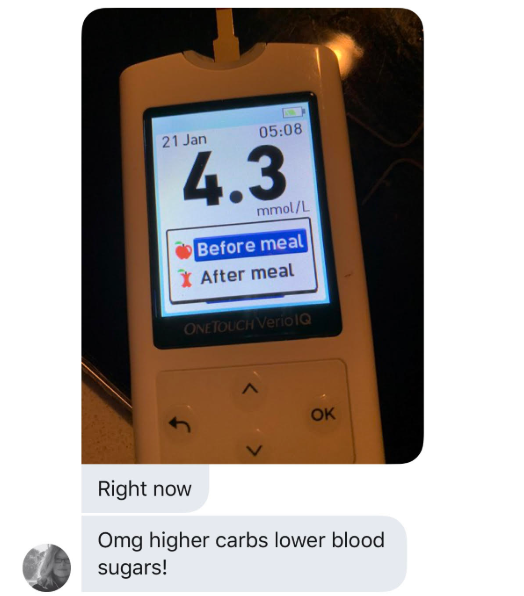
What could be happening here?
Hyperlipid to The Rescue
As usual, an answer could be found in a post by Peter over at Hyperlipid. As usual, it requires some explaining. The post (the second part of it) is about an experiment using “perfused rat pancreas” to study the effects on insulin secretion of several different fats when glucose rises. The pancreas is the organ that releases insulin. “Perfused” means that a solution is pumped through the organ at a steady rate. So you have an isolated rat pancreas, which is being pumped with a buffer solution that contains one of several different fats and a baseline amount of glucose. You suddenly increase the amount of glucose and monitor how much insulin is released by the pancreas. Here’s what happened:
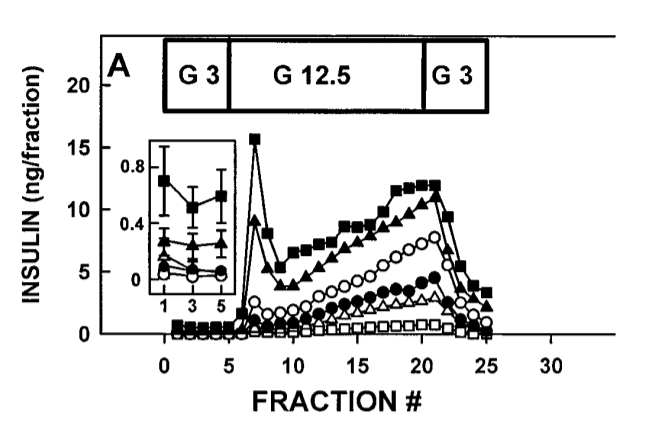
So what’s happening here? (it’s probably easiest to ignore the inset box floating above fractions 0-5). The pancreas’ all have access to a single type of fat. The solid Black Squares are stearic acid (18 carbon length saturated fat), solid triangles are palmitic acid (16 carbon length saturated fat). The open circles are Oleic acid (18 carbon length monounsaturated fat, like in olive oil)., solid circles are linoleic acid (18 carbon length Omega-6 polyunsaturated fat like in soybean oil). Open triangles are Octanoic (8 carbon length saturated like in MCT oil/cocnut oil). The open squares are no fat.
The block of time marked “G 3” is when glucose is held steady at 3 mmol/l. The glucose is suddenly raised to 12.5 mmol/l, “G 12.5”, after fraction 5 and there is a sudden spike in insulin release. Depending on the background fat. As you can see, the longer the carbon chain and the more saturated the fat, the more dramatic the first insulin spike. I’ll let Peter take it from here:
Notice the marked but transient spike in insulin when glucose is raised from 3.0mmol/l to 12.5mmol/l, most obvious in the black squares representing stearic acid as the background FFA (palmitate is the black triangles). After the spike, which I think represents the first phase insulin response, there is a steady climb in insulin, equivalent to the second phase of insulin secretion. …
The surge of insulin would hit the liver and interact with the insulin receptor. Two things follow on from this. Most insulin would be metabolised following interaction with its receptor, so insulin would never flood the systemic circulation. Second effect is that insulin/insulin receptor activation would shut down hepatic glucose output while the Glut2 transporters continue to pretty well clear the portal vein of glucose.
So a first phase insulin response is designed to protect the systemic circulation from both hyperinsulinaemia and hyperglycaemia. That is its job.
Petro Dobromylskyj, https://high-fat-nutrition.blogspot.com/2016/04/dairy-and-diabetes.html
Let’s unpack this. We’ll need to understand what the portal vein is and what the liver’s role in this is. We’ll need to understand the importance of the first-phase insulin response. And what exactly is GLUT2?
The Portal Vein, The Pancreas and The Liver
The portal vein is the highway that connects our digestive organs – the stomach, and intestines – to the pancreas and the liver. As you can see from the illustration, the pancreas – the organ which produces insulin and its counterpart glucagon – sits at the center of the network. The direction of blood flow in all of these veins is from the stomach or intestines past the pancreas and then into the liver.
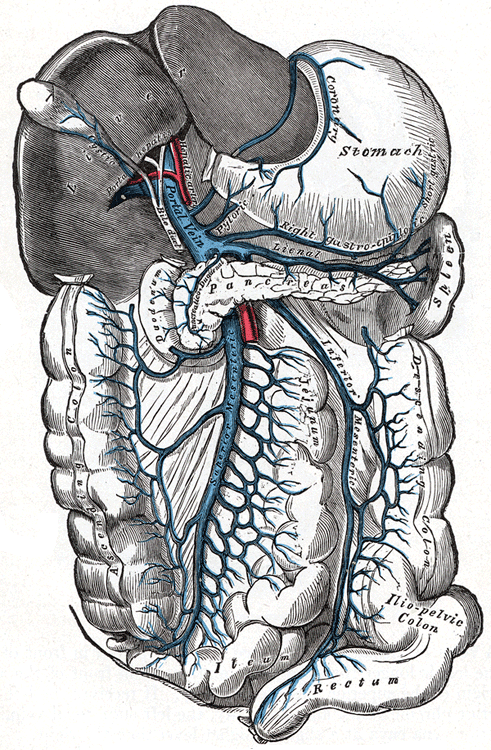
The portal vein feeds into the arterial beds of the liver. Which is to say that the liver gets the first shot at everything coming through the portal vein, which is all of the nutrients extracted by the digestive system. The liver can remove glucose from the portal vein flow or add glucose to it via the action of a glucose transporter called GLUT2. Blood from the portal vein exits the liver through the hepatic veins, which carry the flow to the heart where it is pumped to the rest of the body.
GLUT2 (GLUcose Transporter type 2) is expressed by both the pancreas and the liver. GLUT2 is one of the transporters that can move glucose out of the blood and into the cells WITHOUT being stimulated by insulin. The presence of GLUT2 in the pancreas allows the pancreas to sense glucose coming in through portal flow. GLUT2 has some interesting properties compared to other glucose transporters – it can transport both glucose and fructose, it is capable of transferring large quantities of sugars and it is bidirectional, allowing the liver to export glucose as well as absorb it.
The First-Phase Insulin Response
Let’s look at the perfused rat pancreas again:

When the glucose levels entering the pancreas increases – time period “G 12.5” -the pancreas immediately releases a burst of insulin into the portal vein. Fractions were taken every two minutes during the experiment so fraction 5 is ten minutes in. You can see that the insulin levels peaks in fraction 7, four minutes after glucose rises, and then drops to a significantly lower level than the peak by fraction 9, 8 minutes after the rise in glucose. The times don’t change much if you are eating a starchy meal. Enzymes in your mouth begin converting starch to glucose immediately. The first-phase insulin response happens in the first ten minutes of a meal.
The liver expresses insulin receptors in its capillary beds which bind the insulin coming through the portal vein. The liver’s immediate response to seeing this first blast of insulin is to shut down glucose production. The liver can provide blood glucose by breaking down its stored glycogen in a process know as glycogenolysis or by creating new glucose from glycerol, glucogenic amino acids, pyruvate, lactate and/or some fats in a process known as gluconeogenesis. Either way, the first-phase insulin response tells the liver to stop releasing blood glucose because glucose is coming in from the diet and it’s now time to start storing it as glycogen.
When the liver binds insulin, it rapidly degrades it with an enzyme known as Insulin Degrading Enzyme (IDE). So the first-phase insulin response should be a quick burst of insulin released by the pancreas in response to an increase in glucose in the portal vein. It should shut down glucose production by the liver and trigger the liver to begin rapidly taking up glucose through GLUT2 and storing it as glycogen. The relatively small amount of insulin should be rapidly degraded by the liver and have little effect on systemic insulin.
As you can see, the first-phase insulin response is markedly decreased if the primary fat available to the pancreas is monounsaturated fat (open circles) and it’s almost totally eliminated by linoleic acid (black circles), the polyunsaturated fat found in dietary sources like soybean, safflower and corn oil.
The First-Phase Insulin Response and The Croissant Diet
The liver acts as a sort of metabolic flywheel. It takes incoming glucose, warehouses it as glycogen and then starts to let it back out as blood glucose when needed. The liver can’t store a lot of glycogen – maybe a half lb – and so it acts more like a flywheel than a real energy storage mechanism. But the liver CAN store enough glycogen to absorb the glucose from most meals.
Look at the first tweet from Toshi Clark. The CGM he refers to and the readout he posts is from a Continuous Glucose Monitor. He eats most of a large plate of gnocchi with cream sauce at 6:30 PM (almost on the left hand side of the chart). If anything his blood glucose goes DOWN as a result of this meal due to lowered liver glucose production. Where did the dietary glucose go? Into his liver as glycogen. He clearly has a robust first-phase insulin response. I know from his twitter feed that he’d been eating a diet very high in saturated fat leading up to this plate of gnocchi. Of course we don’t know the total grams of starch from his meal, but a half lb of dry pasta ( most boxes of pasta sold in the US are a pound) only contains 160g of starch, well within the range the liver can store as glycogen. A half lb is a LOT of pasta! (I’m not suggesting he ate that much.)
The other tweets are people reporting lowered fasting blood glucose. As far as I know they were coming from very low carb backgrounds and had their fasting blood glucose lowered due to increasing saturated fat, lowering unsaturated fat and adding back some starch. Most people on a very low carb diet will be glycogen depleted and stay in a more of less constant state of gluconeogenesis to supply the necessary blood glucose. I’m not saying this is a bad thing, and I’m speculating here, but here is my guess as to why The Croissant Diet could lower fasting blood glucose compared to very low carb: by reintroducing some starch in the presence of high saturated fat/absence of PUFA, you re-establish your first phase insulin response. The liver again begins to act as a metabolic flywheel. Gluconeogenesis can be turned off much of the time which results ultimately in lower fasting blood glucose.
I have no proof of this, it’s a guess. It will definitely be an area of future research and speculation.
The Loss of the First Phase Insulin Response is a Bad Thing
Interestingly, loss of the first-phase insulin response is an early warning sign that someone is on the road to Type 2 diabetes. From this review paper:
It is widely thought that diminution of first-phase insulin release is the earliest detectable defect of β-cell function in individuals destined to develop type 2 diabetes
Is Reduced First-Phase Insulin Release the Earliest Detectable Abnormality in Individuals Destined to Develop Type 2 Diabetes?
John E. Gerich
Diabetes Feb 2002, 51 (suppl 1) S117-S121; DOI: 10.2337/diabetes.51.2007.S117
This makes sense when you think about it. The first phase insulin response is the thing that tells your liver to stop releasing blood sugar and start absorbing it. If after a meal your liver is happily releasing glucose while glucose is being produced in the portal vein, that sounds like a recipe for high post-meal blood glucose. It seems that the loss of the first-phase insulin response is the first sign that your insulin/glucose system is becoming dysregulated.

I cannot see the graph from Toshi Clark.
I eat mainly keto but have tried some high stearic acid mashed potatoes after my workouts. Unfortunately, I did not have a CGM. I may have to invest again in the CGM to see what happens.
I have a pin-prick meter, though, which I use only in the mornings. There was a lowering the next day after trying potatoes at lunch, but the error in these are too high to use one data point. I’ll have to get a CGM again.
I am conflicted about blood sugar for those of us who are keto. For instance, if I work around the house (think going up and down 2-3 flights of stairs many times), when I was wearing my CGM, I consistently got a higher blood sugar those days, 10+ points higher then when I was at work without any morning workout. If I work out in the mornings (body weight training to failure, HIIT), my blood sugar goes up and the daily value will be higher than on those days, too. And if I combine the two (morning workout, working around the house), it’s even higher.
I think this is the body trying to get glycogen for your muscles from somewhere. (It’s why I hypothesize that Shawn Baker’s HbA1c is so high–something has to fuel his workouts.) It’s unclear to me that’s a bad thing.
So, is there a benefit to lowering blood glucose using stearic acid and starch? To me, this is unclear. To the extent the muscles need glycogen, perhaps at least some of the carbs are being shuttled there? (This is the reason for my eating starch after a workout.)
I’m just not sure how relevant the data point of “fasting” blood sugar is. (And we should define “fasting” blood sugar; I usually think of this as morning, fasting blood sugar.)
By the way, I heard a podcast with DR. Ben Bikman. He theorized that one possible reason you got (and I seem to get) a benefit was because stearic acid is preferentially burnt as fuel and not stored, whereas palmitic acid is preferentially stored.
Can you see the tweet itself?
https://twitter.com/maximumcharacte/status/1221829221141467136
I don’t necessarily mean to imply that low fasting blood glucose is necessarily a very important objective. But I DO find that many people reporting well below average FBG while adding starch to a high Sat Fat diet is INTERESTING. It’s not what I expected and it’s been rattling around in my head since I read Peter’s post.
I heard Ben Bikman’s podcast. It’s unclear to me why he believes that stearic is preferentially oxidized. This acrticle:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3588585/
shows a bunch of tracer studies done with various dietary fats and it’s clear that shorter chain fats and more unsaturated fats are oxidized preferentially. So lauric acid (12 carbon saturated) burns faster than linolenic (18 carbon triple unsaturated) burns faster than linoleic (18 carbon double unsaturated) burns faster than oleic (18 carbon monounsaturated) burns faster than palmitic (16 carbon saturated) burns faster than stearic (18 carbon saturated).
possibly, the shorter chain FA’s burn more quickly as it take less energy to oxidize?
If you are eating low carb/high fat and are regularly physically active, your skeletal muscles will utilize free fatty acids for energy, and only glycogen for short, anerobic bursts of activity.
Maybe this was from hyperlipid also;the first phase insulin is also vital for supressing glugacon -and keep it in leash afterwards with lesser insulin (stearic and palmitic curves do that).
Unger showed that glucose disappears from blood without insulin -it takes a bit longer I guess.
The role of insulin is to clean and store energy from blood -stop lipolysis first and pave the way for glucose burning/storing. Shove FFA’s away into storage.
So “traditional” FFA’s spike the insulin reaction, the “new” FFA’s do not. hmmm, this seems to have greatest effect in the liver.
Needs some thinking…
JR
When you say “the role of insulin” I think you’re talking about systemic circulation. Clearly the role of insulin in the first phase response is to reduce liver glucose production and increase glycogen storage.
few why’s q’s:
1) what causes SFA to have such dramatic effect? more glucose left in the blood (cells not taking up glucose because they are ROSing left and right or is it something else?)
2) wouldn’t this somehow “wear out” pancreas in the logn run? higher blood glucose in the rest of the system is obviously bad but hitting you pancreas with such huge glucose malus can be as bad.
1) Presumably the release of insulin is due to ROS production in the pancreas in the presence of glucose, but I think this is speculative.
2) I don’t think so, I think this is how it’s supposed to work.
Brad, If I remember my anatomy correctly, the pancreas is fed by arterial circulation and drains into the portal vein, then to the liver. The pancreas does not to my knowledge see portal vein blood coming from the intestines.
By the way, exogenous insulin administered subcutaneously bypasses the liver. Much higher blood levels are therefore needed, worsening hyperinsulinemia in type 2 diabetics.
I’m not a phsyiologist and its possible that I have things slightly off. The pancreas may draw most of its blood flow from the abdominal aorta but the portal vein runs THROUGH the pancreas and the pancreas DEF puts out a burst of insulin within 10 minutes of consuming starch.
And yes, I suspect that administering insulin subcutaneously is a poor substitute for a robust firt-pass insulin response.
I had to look this up and it’s not easy. but pancreas venous drainage primarily to portal vein from various branches that serve the pancreatic head and the body/tail, separately. Drainage not always direct, but through splenic and superior mesenteric vein.
https://www.youtube.com/watch?v=tLIRHF_kxZE
What I find surprising in such articles is that I thought insulin sensitivity, insulin resistance is something muscle cells do. You are saying also fat cells do it.
This weirds me out. Like Peter saying insulin sensitivity makes you fat. But “traditionally” insulin sensitivity is a property of muscle cells. I go to the gym and bench 80 kg fifty times. My muscles are emptied of nutrients. So they are sensitive to insulin. So I go eat a cheesecake and not get fat because insulin sensitivity stores it in the muscles. Clearly a good thing.
I am not sure what is going on here…
Actually most (all?) cell types can respond to insulin. Or not.
insulin sensitivity in adipocytes -> fat storage and insulin-induced suppression of free fatty acids
insulin sensitivity in liver -> reduced hepatic glucose output
insulin sensitivity in skeletal muscle -> clearance of blood glucose
Nevertheless I am willing to try to try Brad’s ideas in my diet. This is the first time I am going to try a specific diet in decades. Yes I am fat.
My opinion is that the *psychological* reason we are fat is not that we like bad food and do not like good food. In my opinion, it is that we want easy, convenient and socially normal food.
So my version of Brad’s diet is a little bread, a lot of butter and some cheese. Mostly for variety – there is a big variety in cheese. And eggs, and steaks.
That is because bread and butter is the most “normal” food as “ones daily bread and butter”. It is very obvious. In a way that candle croissants are not.
That is, the “experts” are exhorting us to be conscious in our diets. Like to be conscious about the environment etc. But my view is that we are fat because we do not want to be conscious. I do not. I do not want to pay attention to eating. So I think 35g bread (half a Kaisersemmel here in Austria), 100g butter (just eyeballing a 250g brick cutting it somewhere below half), and a thick slice of whatever cheese is going to do it when I do not feel like whipping out a pan to make steak or cheese. When I do, I do. Bread and butter is something you can do entirely unconsciously.
I respect people who go on “diets” that are restricted in terms of the social and other pleasures associated with “eating”. However, I agree that expanding the possibilities is a good idea…if you stick to certain principles. The French and other European countries have developed their palates and love for SFA. SFA and starch hits my dopamine and probably does not screw me up …until, of course, too much N6 PUFA sneaks in the back door. Recently I ate a pretty big plate of very creamy fettucine alfredo. Hardly budged my BG more than 10-15 pts. How it did that is being discussed. Yes, I felt very satiated.
The more I see, it seems that the carbs from fettucine alfredo are handled very differently than, for instance, Coca Cola.
>In my opinion, it is that we want easy, convenient and socially normal food.
I mean, Brad is a chef. He is interested in food. But most people aren’t. I am not. When I was 16 my parents gave me lunch money and I usually got 4 McD cheeseburgers not because I liked them that much but that was the most easily obviously convenient way to stop being hungry without paying any attention to it, because my brain was occupied with entirely different things. I just inhaled them without any paying attention.
This is why I think to use Brad’s ideas modified as bread and butter – a little bread, lot of butter. This is yet another kind of no-brainer food, similar to McD.
One more aspect…
Prof. Noakes referred to this baboon study during his trial presentation. The measurements were done directly in the portal vein…! Cannot be done in humans.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3566507/#__ffn_sectitle
What he found most interesting, was that the liver glucose production (gluconeogenesis) never stops completely. It reduces quite much in HCLF feeding vs. LowCHF (9 vs. 15 units). The systemic circulation sees >30 vs <20 units of glucose for 4-5 hours after these meals.
This explained to him, why moderation in carbs is essential to insulin resistant or prediabetic people. Not so for those who have normal response to carbs.
JR
That IS a very interesting study. Thanks for posting.
interesting experiment…I have been eating lots of roast potatoes with butter and…have actually managed to gain quite a bit of weight. I don’t think it works for everybody. I seem to lose weight eating more olive oil. I am insulin resistant prediabetic. No veggies oils in…a VERY long time apart from olive oil. I did keto for years, gave it up during pregnancy and did blood type diet with just olive oil no butter. I seem to be one of the few that actually get hungrier with butter and starch together…. and really keeping n-6 to a minimum. Just another anecdote here.
Hi! Thanks for your feedback. This approach of course hasn’t worked for everyone (do any?), but it certainly seems to be working for quite a few. I suspect it depends on your level of metabolic flexibility. I suspect it would not have worked for me had I not supplemented with the extra stearic acid.
I think you can improve metabolic flexibility partly forcing it by diet. However, if you can do some HR zone 2 training and increase your aerobic base by burning more fat and less glucose, it can wake up that metabolic machinery. Just my 2 cents. Weight training with barbells got me stronger but I just go more hungry and gained weight not proportional to muscle gain.
Can someone please link to a good primer on insulin? The more I read about it, the more confused I get.
Ha! I don’t think you’re the only one who’s confused. Unfortunately, most primers on insulin are thinking about it very differently so I’m not sure how helpful they’ll be in figuring out the theories presented here.
I had to look this up and it’s not easy. but pancreas venous drainage primarily to portal vein from various branches that serve the pancreatic head and the body/tail, separately. Drainage not always direct, but through splenic and superior mesenteric vein.
https://www.youtube.com/watch?v=tLIRHF_kxZE
you could not store an extra 160 gms of carbohydrate in your liver. The liver is not an empty fly wheel. It is more like a back up… yes, it can hold 160 gms, but it will never get that empty. Under almost any normal conditions, it will never get down to 300 calories…. It ALWAYS keeps some in reserve for fight or flight, Life or death situations where someone has to lift a car off of a baby. Or run away from a lion in fear of your life. it takes a massive dose of hormones to get the liver to drop that much energy. it is a buffer. When you start dropping more than 50 calories (Plus you are using some, not all, of the muscle glycogen) your luver kicks up production. even on a zero carb diet, muscle and liver glycogen is the same….. it is not some empty flywheel. It is a homeostasis, the same place where it would be eating carbs.
How much stearic acid is in the 7.5-oz. Butter Oil product you are selling? Label shows the ingredients but not quantity of stearic acid per serving, for instance.
It is about 27% stearic acid by weight.